Effective Ways to Calculate Percent Yield for Modern Chemistry Practices in 2025
Understanding how to calculate percent yield is vital for chemists and students alike, as it provides insight into the efficiency of chemical reactions in the lab. This article explores the fundamental concepts and practical applications of percent yield calculation, including its formula, definitions, and factors that can affect results. Whether you’re an experienced chemist or a student just starting with laboratory practices, mastering percent yield will enhance your experimental outcomes.
Understanding Percent Yield in Chemistry
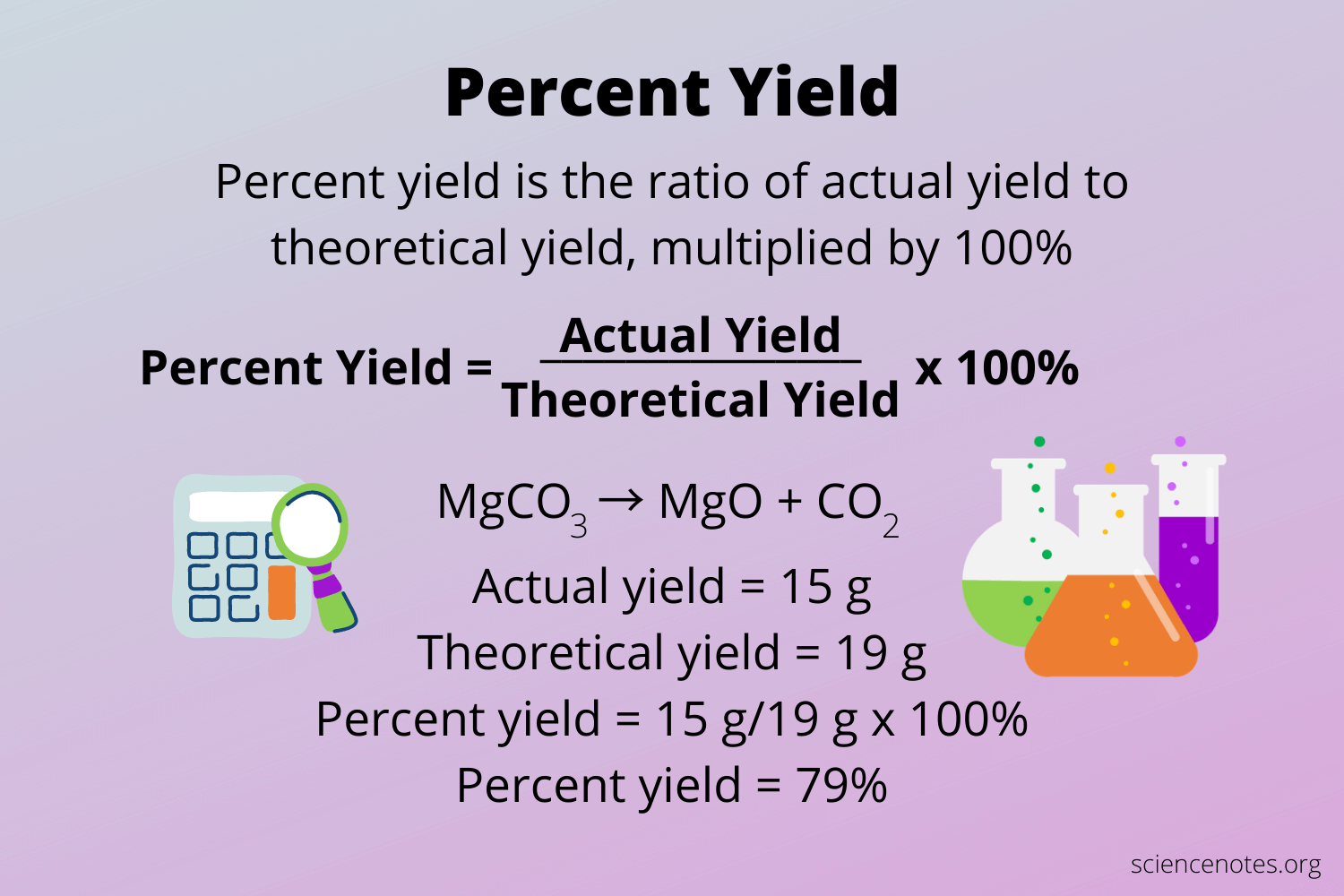
Percent yield in chemistry is a crucial metric for evaluating the success of a chemical reaction. The percent yield definition refers to the ratio of the actual yield of a product obtained from a chemical reaction to the theoretical yield, expressed as a percentage. The actual yield is the quantity of product one actually obtains, while the theoretical yield calculates what one could theoretically achieve if the reaction goes perfectly according to stoichiometric principles. Understanding percent yield helps in analyzing actual yield vs theoretical yield, ultimately improving experimental protocols.
Percent Yield Formula and Calculation
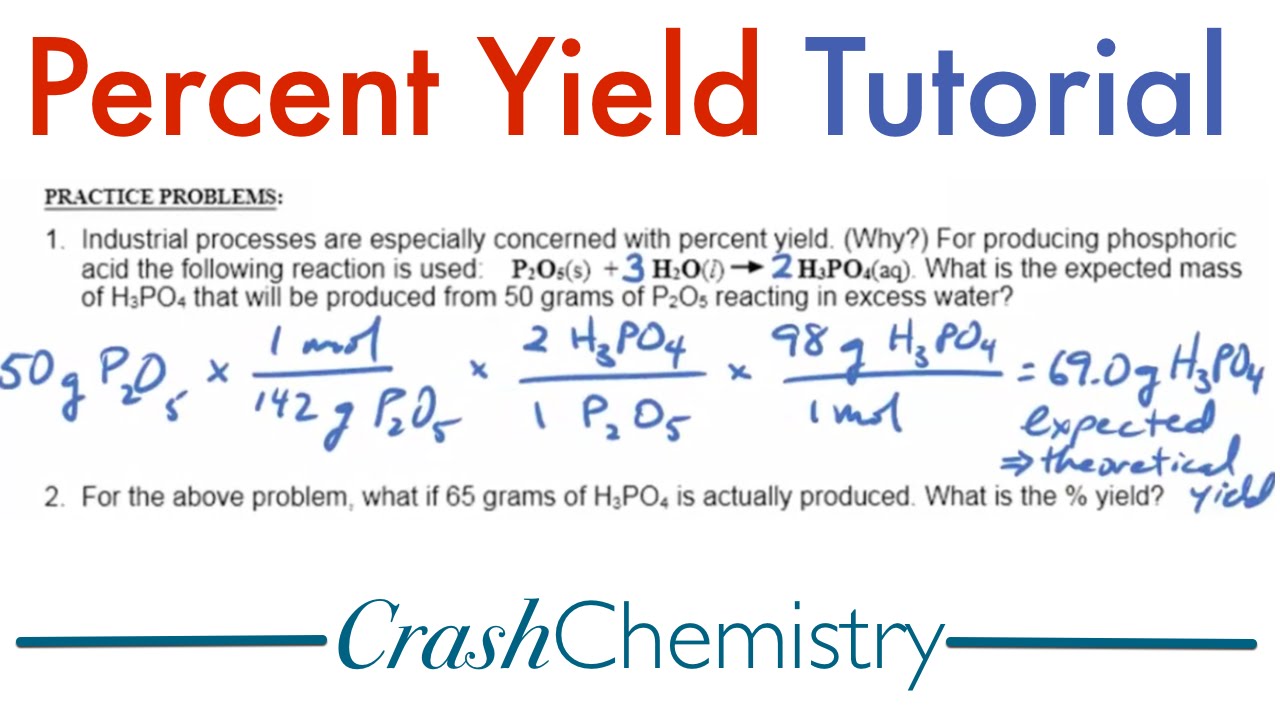
The percent yield formula is straightforward yet vital in laboratory settings. To determine percent yield, use the following equation:
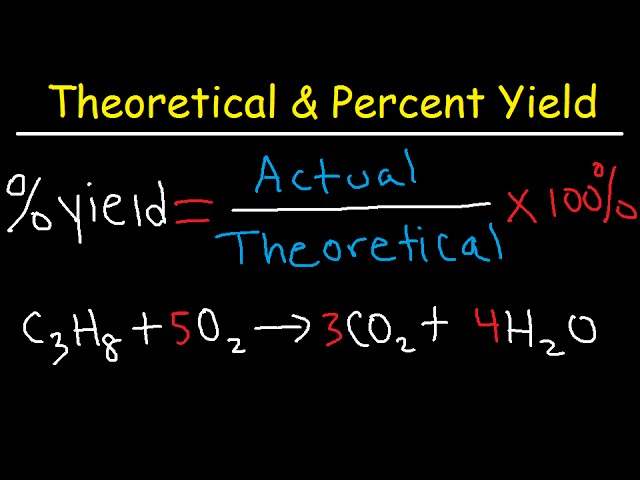
Percent Yield = (Actual Yield / Theoretical Yield) × 100.
This formula allows chemists to quickly assess the effectiveness of their reactions. For example, if an experiment predicts that 10 grams of a product should form but only 7 grams are collected, the percent yield calculation would be (7g / 10g) × 100 = 70%. Using this percentage, chemists can evaluate if conditions were favorable or if adjustments need to be made in future experiments.
Factors Affecting Percent Yield
Several factors can influence chemical reaction yield, notably impacting the percent yield interpretation. These include reaction conditions, purity of reactants, and procedural errors. For instance, temperature fluctuations or incorrect proportions of reactants may lead to incomplete reactions, lowering the actual yield. Additionally, evaporation or side reactions can contribute to yield loss. Understanding these factors affecting percent yield can help researchers strategically optimize their processes, leading to more reliable and accurate results.
Improving Your Percent Yield
Every chemist aims to improve their percent yield in chemistry to ensure maximum conversion of reactants to products. There are various strategies one can adopt to achieve this. First, standardizing procedures can minimize variability and errors. Additionally, understanding the theoretical yield formula can assist chemists in calculating realistic expectations for their reactions. By meticulously measuring and controlling experimental conditions, researchers can ensure that they are achieving the best possible outcomes from every reaction.
Common Mistakes in Yield Calculation
When dealing with yield calculations in experiments, mistakes often lead to inaccurate percent yield percentages. Some common pitfalls include miscalculating or misrecording the actual or theoretical yields, which can occur due to simple error or oversight. Another frequent issue is the improper understanding of the purity of reactants, where using materials with impurities can skew yield values. It is essential to develop robust systems and checklists to prevent these errors, ensuring valid percent yield calculations and leveraging them to enhance experimental outcomes.
Implementing Process Optimization Techniques
To further increase percent yield, employing optimization techniques in chemistry becomes necessary. Techniques such as adjusting reaction times, temperatures, or concentrations can provide insights into maximizing the reaction efficiency. For example, running a reaction under varying temperatures can reveal how heat affects product formation, allowing you to select the ideal conditions to boost your yield. Additionally, utilizing tools like statistical process control can help in improving yield efficiency based on empirical data.
Percent Yield Examples in Laboratories
Exploring practical percent yield examples in labs is crucial for better conceptual understanding. In one laboratory experiment, a chemist performs a synthesis reaction aiming to create aspirin. The theoretical yield calculated indicates a potential of 5 grams, but lab conditions yield only 4 grams of aspirin. By applying the percent yield formula, the chemist finds that (4g / 5g) × 100 = 80%. This information is beneficial for evaluating the reaction’s efficiency and determining which adjustments may be needed for improved results in subsequent attempts.
Experimental vs Theoretical Yield: A Case Study
Another fascinating case involving experimental vs theoretical yield deals with the synthesis of sodium chloride from sodium and chlorine. Theoretical calculations predict a yield of 10 grams under perfect conditions, but due to factors such as sodium loss in handling and reaction inefficiencies, only 7.5 grams are actualized. The percent yield, calculated as (7.5g / 10g) × 100 = 75%, highlights significant areas for process improvement. This analysis encourages chemists to revisit their methodologies, enhancing the understanding of processes that could lead to maximizing yield.
Assessing Overall Yield Impact
Understanding how different factors contribute to yield loss is critical in laboratories. Evaluating yield loss factors will help define best practices, such as ensuring consistent stoichiometric ratios are maintained during reactions. By creating a feedback loop where data analysis influences procedural changes, chemists can assess the overall yield impact and progressively enhance their techniques.
Key Takeaways
- Percent yield calculation is fundamental for assessing reaction efficiency.
- Identifying factors affecting yields aids in improving laboratory practices.
- Implementing optimization techniques and analyzing common mistakes can boost yield percentages.
- Real-world examples are invaluable for mastering the interpretation of percent yield.
FAQ
1. What does percent yield mean in chemistry?
Percent yield refers to the ratio of the actual yield of a product obtained from a chemical reaction to the theoretical yield, expressed as a percentage. It is used to evaluate the efficiency of a reaction, helping chemists determine how effectively reactants are transformed into products.
2. How can I improve my experiment’s percent yield?
Improving your experiment’s percent yield can be achieved by optimizing reaction conditions, ensuring high-quality reagents, limiting evaporation, and adjusting procedural steps to minimize losses. Consistent methodologies and thorough documentation of results also facilitate enhancements.
3. What is the formula for calculating percent yield?
The formula for calculating percent yield is: Percent Yield = (Actual Yield / Theoretical Yield) × 100. This gives chemists a clear indication of the efficiency of their reactions.
4. What are common mistakes made in yield calculations?
Common mistakes in yield calculations include incorrect measurements of actual and theoretical yields, overlooking reactant purity, and failing to consider side reactions that can affect outcomes. Proper laboratory practices can minimize these errors.
5. Why is percent yield significant in chemical reactions?
Percent yield is significant because it allows chemists to gauge the efficiency and effectiveness of their experiments. It helps identify areas for improvement, validate methodologies, and maximize resource utilization, leading to better experimental design and process application.
6. What are theoretical yield and its importance?
Theoretical yield is the maximum amount of product that could form from given reactants in a chemical reaction, based on stoichiometry. Its importance lies in providing a benchmark for comparing actual yield, helping chemists understand the completeness of a reaction.
7. How do factors affect percent yield?
Factors influencing percent yield include reaction conditions, reactant purity, the efficiency of mixing, and potential side reactions. By controlling these variables, chemists can enhance yields and improve their overall experimental results.