Effective Ways to Calculate Percent Yield in 2025: Mastering Chemistry Techniques
Understanding percent yield is essential for anyone involved in chemistry, from students to seasoned researchers. This vital concept helps chemists determine the efficiency of a reaction by comparing the amount of product actually obtained to the theoretical possible yield. By mastering the techniques of calculating percent yield, you can enhance your laboratory practices and optimize your results. In this article, we will explore various ways to effectively calculate percent yield, including utilizing the percent yield formula and addressing common challenges.
Understanding Percent Yield in Chemistry
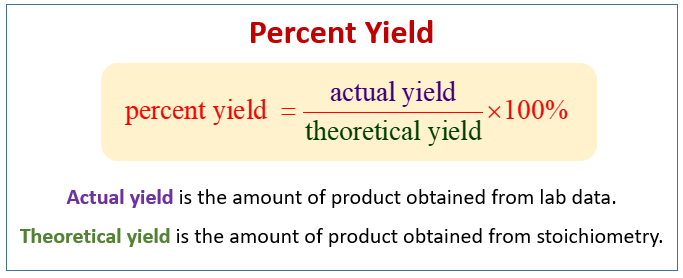
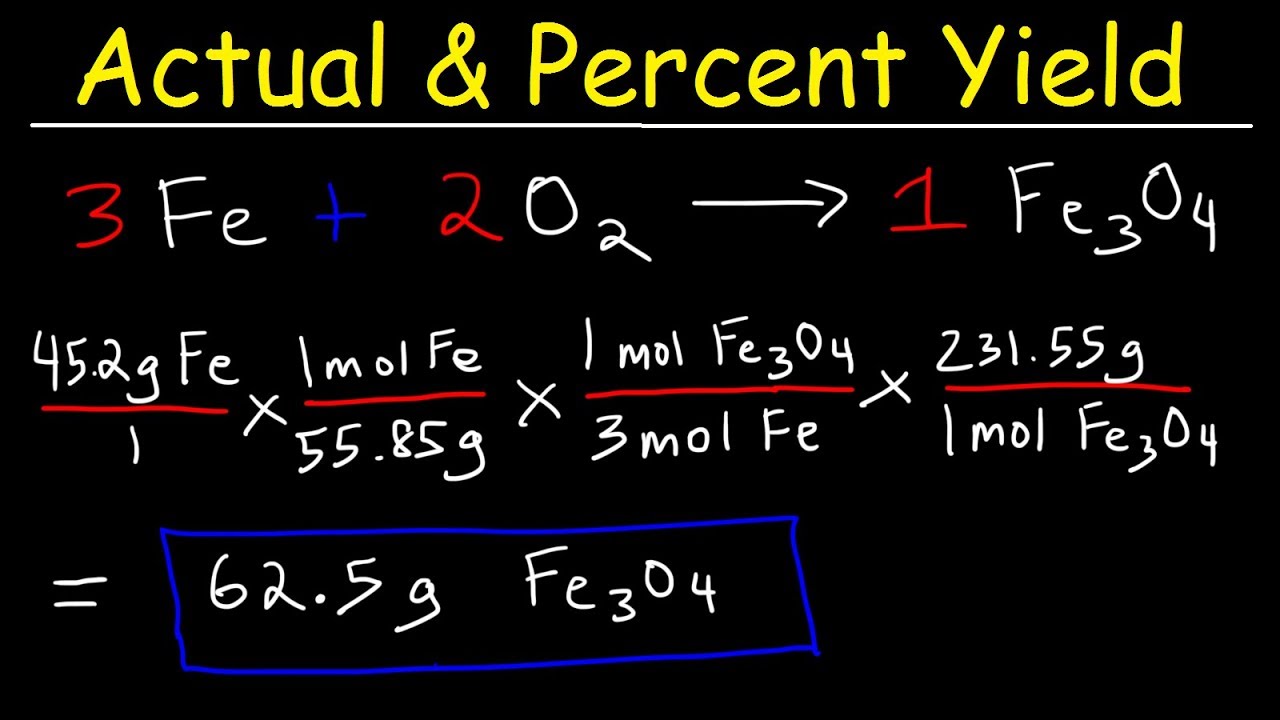
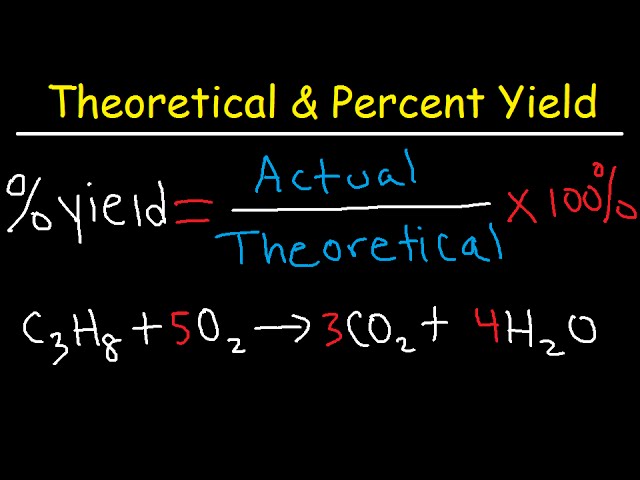
Percent yield is defined as the ratio of the actual yield obtained from a chemical reaction to the theoretical yield predicted by stoichiometry, expressed as a percentage. The percent yield equation is formulated as: % Yield = (Actual Yield / Theoretical Yield) x 100. This metric is crucial in percent yield chemistry as it allows chemists to evaluate the success of their reactions and experiments. Whether in an academic setting or a professional lab, grasping the significance of percent yield is indispensable for improving yield efficiency.
Theoretical vs Actual Yield
Understanding the difference between actual and theoretical yield is fundamental in assessing reaction yield. The theoretical yield is the maximum amount of product that can theoretically be formed from a given amount of reactants under ideal conditions. Conversely, the actual yield is what you truly obtain at the end of a reaction. Many factors, such as incomplete reactions, loss of product during recovery, and side reactions, can lead to discrepancies between these two yields. Therefore, professionals must continuously evaluate these factors when calculating yield in chemistry.
Factors Affecting Percent Yield
Several factors influence percent yield. For instance, reaction conditions like temperature, pressure, and concentration can significantly affect how well reactants convert to products. Additionally, the purity of starting materials and effective stirring or mixing can improve overall yield. Managers can optimize yield significantly by controlling these parameters. Employing appropriate methods to classify and record these yields becomes crucial for accurate yield determination in experiments.
Strategies for Improving Percent Yield
Maximizing yield efficiency in a laboratory often requires practical strategies and careful adjustments. One approach is to ensure every step of your reaction is optimized, from selecting the correct reagents to precise temperature control during the reaction. Applying cross-sectional studies or comparative analyses can also provide insights for optimized yield calculations in experiments. Attention to detail in technique can drastically influence your overall yield percentage.
Maximizing Yield in Organic Chemistry
In organic chemistry, maximizing yield can sometimes be achieved through modifications in solvent choice or reaction technique. For example, utilizing reflux techniques often allows for more time for reactions to occur, potentially leading to a higher rate of conversion from reactants to products. A practical example of optimizing yields is using a separatory funnel to minimize product loss during extraction. This can significantly contribute to your percentage yield in practicals.
Common Mistakes in Yield Calculation
Common errors in yield calculation can stem from misreading measurements or using impure substances as starting materials. Incorrectly estimating the theoretical yield due to stoichiometric miscalculations can mislead your final percent yield calculation. A clear understanding of these potential pitfalls and systematic practices can enhance the accuracy of your yield analysis.
Advanced Percent Yield Calculations
For experienced chemists, more advanced techniques such as statistical analysis methods can be beneficial. Robust analyses of previously recorded data help in achieving high yield ratios. Utilizing software tools to model reaction kinetics can also assist in predicting better yields and understanding complex chemical dynamics. Being adept in these methods not only improves lab productivity but ensures consistent accuracy in yield reports.
Yield Analysis Techniques
Applying sophisticated yield analysis techniques helps in effectively comparing yield metrics across different setups or experiments. For instance, utilizing comparative methods like the t-test allows researchers to evaluate the effectiveness of different reagent combinations reliably. Moreover, carrying out repeated trials and calculating averages can also help achieve more precise laboratory yield percentages.
Utilizing Data Analysis for Performance Yield Indicators
In any scientific endeavor, compiling and analyzing data critically remains essential for understanding overall performance. By documenting all aspects of yield performance, from reaction rates to final yield determination, scientists can identify trends and make informed decisions. Incorporating such evaluative measures can enhance both the integrity and efficiency of chemical research. This supports a solid foundation for accurately assessing reaction yield and ensuring high-quality results.
Key Takeaways
- Percent yield is a critical measurement for determining the efficiency of chemical reactions.
- Understanding the difference between actual and theoretical yield is essential for accurate assessments.
- Multiple factors can affect yield; optimizing laboratory conditions can enhance outcomes significantly.
- Implementing effective analysis techniques and strategies contributes to better yield performance in experiments.
- Common mistakes can be minimized through careful handling of procedures and materials.
FAQ
1. What is the importance of understanding percent yield in experiments?
Understanding percent yield enables chemists to evaluate the effectiveness of reactions and optimize conditions for better outcomes. This metric provides information on efficiency, helping to adjust reagents and processes accordingly.
2. How can one improve percent yield calculations in organic chemistry?
Improving the accuracy of stuff can involve various techniques like optimizing the reaction environment, utilizing high-purity reagents, and employing advanced characterization methods during the product recovery and purification processes.
3. What common errors occur in yield calculations?
Miscalculation of the theoretical yield, measurement errors, and interpreting reaction mixture compositions incorrectly can lead to significant errors in yield calculations, affecting research outcomes.
4. Can external factors influence yield percentage?
Yes, fluctuations in environmental conditions such as temperature, pressure, and reagent concentration significantly impact the efficiency and therefore the yield percentage of chemical reactions in the laboratory.
5. What strategies can be used to maximize yield ratios?
Implementing strategies like optimizing reaction pathways, refining separation techniques, and ensuring precise reagent quantities can maximize yield ratios, as well as conducting iterative experiments based on previous findings to enhance outcomes.
Images:
For additional insights, feel free to explore relevant topics on calculation methods and practical applications.