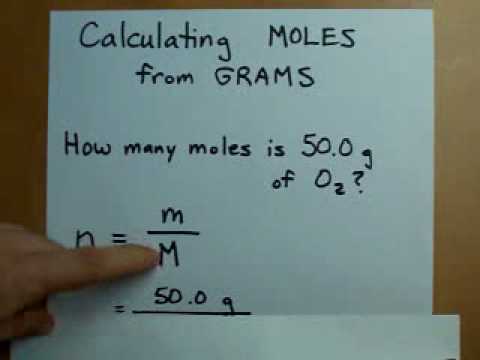How to Properly Calculate Moles in 2025: A Smart Guide to Mole Conversions and Chemistry Fundamentals
Understanding how to calculate moles is essential for anyone studying chemistry. This guide will walk you through the fundamental aspects of mole calculations, mole concept definitions, and the various methods to convert between grams and moles. By the end, you’ll have a clear understanding of the mole, its significance in chemistry, and practical examples to solidify your learning.
Mole Calculation Basics
The mole is the cornerstone of chemistry, serving as a bridge between the microscopic world of atoms and molecules and the macroscopic world of grams and liters. A mole, defined as Avogadro’s number (approximately 6.022 x 1023), allows us to quantify the number of particles in a given sample. When discussing **molar mass determination**, it’s critical to recognize that it is the mass of one mole of a substance. This is typically expressed in grams per mole (g/mol) and can be determined from the periodic table by adding together the atomic masses of all the elements in a compound.
Understanding Molar Mass
**Molar mass** is the mass of one mole of a substance and is a vital component in **calculating moles of a substance**. To find molar mass, sum the atomic masses of the elements in a compound based on its formula. For example, the molecular formula H2O can be calculated as follows: Hydrogen has an atomic mass of about 1 g/mol, and oxygen about 16 g/mol. Therefore, the molar mass of water is 2(1) + 16 = 18 g/mol. Knowing this allows you to convert grams of water to moles quickly.
Mole Calculation Formula
The **mole calculation formula** is straightforward yet essential in understanding how to proceed with conversions. It can be expressed as:
Number of Moles (n) = Mass (g) / Molar Mass (g/mol)
Using this formula, if you have 36 g of water, you can calculate the moles: n = 36 g / 18 g/mol = 2 moles. This formula facilitates many conversions and calculations in chemistry, whether you’re performing a **moles to grams conversion** or looking into **stoichiometry and moles**.
Converting Grams to Moles
**Converting grams to moles** is a critical skill in chemistry, and the process requires an understanding of molar mass as discussed earlier. To make a conversion, you’d first figure out the molar mass of the substance you’re interested in. Once you have this value, you can swiftly use it to convert grams into moles.
Step-by-Step Grams to Moles Conversion
1. **Determine Molar Mass**: Calculate the molar mass of your compound using the periodic table.
2. **Use the Mole Calculation Formula**: Apply the formula mentioned above: Number of Moles (n) = Mass (g) / Molar Mass (g/mol).
3. **Calculate**: Insert your values and solve for moles. For example, if you have 50 g of NaCl, and its molar mass is 58.44 g/mol: n = 50 g / 58.44 g/mol = 0.857 moles of sodium chloride.
Effects of Temperature and Pressure on Moles
Temperature and pressure can significantly impact gas moles in reactions. According to the ideal gas law, one mole of gas occupies 22.4 liters at standard temperature and pressure (STP). If conditions change, such as increasing the temperature, the volume will change, which requires a recalibration of the moles present. Therefore, it’s vital to correctly identify conditions to apply accurate **mole in gas calculations**.
Calculating Moles in Chemical Reactions
In understanding **calculating moles in a reaction**, one cannot overlook the importance of stoichiometry. Stoichiometry involves the quantitative relationships between reactants and products in chemical reactions. With a balanced equation, you can calculate how many moles of reactants are needed or how many moles of products are expected.
Stoichiometry Example
Consider a reaction between hydrogen gas and oxygen gas to form water: 2H2 + O2 → 2H2O. From this balanced equation, you can see that 2 moles of H2 react with 1 mole of O2 to produce 2 moles of water. If you start with 4 moles of H2, you’ll need 2 moles of O2 to completely react, generating 4 moles of water in return.
Limiting Reactants and Theoretical Yield
In many chemical reactions, one reactant may be consumed first, limiting the reaction and determining the maximum amount of product formed, known as theoretical yield. To find the limiting reactant, calculate the moles of each reactant and compare their ratios per the balanced equation. Understanding the concept of limiting reactants is instrumental in optimizing chemical reactions and maximizing yields.
Real-World Applications of Moles
The **significance of moles in chemistry** extends beyond academic exercises; it plays an integral role in everyday applications. For example, in pharmaceuticals, it’s essential to determine the correct moles to ensure dosages are safe and effective. Similarly, in environmental science, understanding the concentration of pollutants can be analyzed using mole calculations.
Applications in Solutions
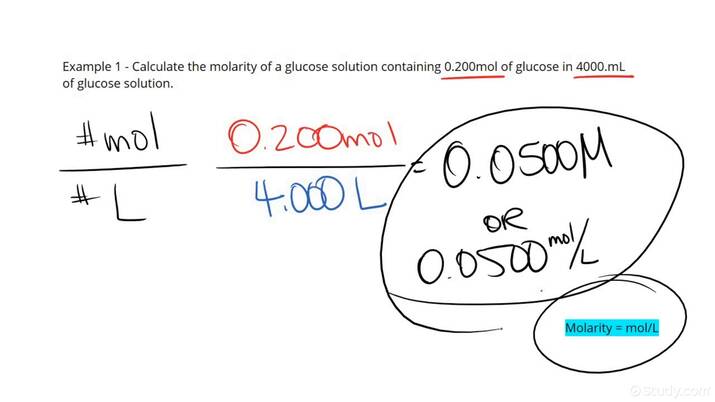
In creating solutions, knowing how to calculate molarity—concentration measured in moles per liter—is vital. This involves using the formula:
Molarity (M) = Number of Moles of Solute / Volume of Solution in Liters
For instance, if you dissolve 2 moles of NaCl in 1 L of water, the molarity is 2 M. This principle is vital in laboratory settings and industry, ensuring precision is maintained in chemical experiments and product formulations.
Teaching and Understanding Moles
Teaching others about the mole concept can be challenging yet rewarding. Utilizing practical examples and experiments can enhance understanding. For instance, allowing students to measure out grams of a substance and then convert to moles during laboratory exercises can elevate their grasp on moles and reinforce the concepts through real-world experiences.
Conclusion
Knowing how to calculate moles is a foundational skill in chemistry that facilitates effective scientific communication and understanding. By mastering the **mole calculation formulas**, converting between units, and applying these concepts in practical situations, you become proficient in the language of chemistry.
Key Takeaways
- The mole is a key measurement in chemistry, linking atomic scale to macroscopic quantities.
- Molar mass determination allows conversions between grams and moles.
- Stoichiometry is essential in understanding the relationships in chemical reactions.
- Moles have practical applications in both laboratory and everyday settings.
- Teaching the mole concept effectively can enhance student comprehension.
FAQ
1. What is the relationship between moles and molarity?
Molarity refers to the concentration of a solute in a solution, measured in moles per liter. This relationship informs how many moles of solute you’ll find in a given volume of solution, facilitating easy conversions in chemistry.
2. How do you calculate the number of moles from concentration?
To calculate the number of moles from concentration, use the formula: Number of Moles = Molarity × Volume of Solution in Liters. This method assists in taking accurate measurements in laboratory works.
3. Can you provide an example of moles in everyday life?
An example would be calculating the moles of salt (NaCl) when cooking, helping you determine the right amount for perfectly seasoned meals while ensuring consistency across recipes.
4. Why is the concept of moles important in chemical reactions?
The concept of moles helps chemists understand the ratios of reactants and products, leading to more productive reactions and accurate formulations in various chemical processes.
5. What common mistakes should be avoided when calculating moles?
Common mistakes include using incorrect molar mass values or failing to balance the chemical equation properly before performing stoichiometric calculations. Always double-check your calculations to ensure accuracy.
6. How can density assist in calculating moles?
Density can be used to convert volume to mass and then to moles by first determining the mass from the volume and density formula (mass = volume × density), then applying the mole calculation formula.
7. What is the significance of Avogadro’s number in calculations involving moles?
Avogadro’s number allows chemists to convert between the number of moles and the actual number of particles (atoms, molecules, etc.), making the quantification of chemical reactions and materials accessible and manageable.